Denosumab for Osteoporosis May Prevent Type 2 Diabetes Denosumab for Osteoporosis May Prevent Type 2 Diabetes
July 2, 2024What Does FDA Approve? Part 2
July 2, 2024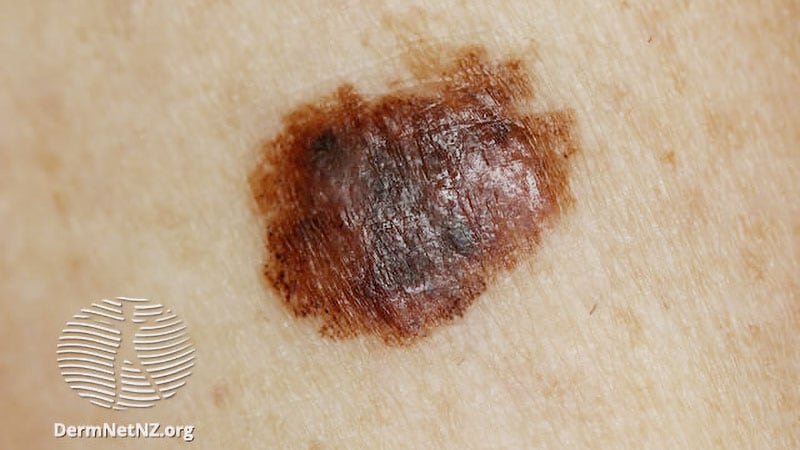
TOPLINE:
In patients with resected stage III melanoma, adjuvant therapy with dabrafenib plus trametinib reduces the risk for death by 20% compared with placebo, though the overall survival benefit does not reach statistical significance. The combination lowers the risk for death by a greater degree — 25% — in patients with a BRAF V600E mutation.
METHODOLOGY:
- The combination of BRAF-targeted dabrafenib and the MEK inhibitor trametinib has become a standard treatment in the adjuvant setting for patients with BRAF V600–mutated stage III melanoma.
- Interim results from the phase 3 COMBI-AD trial found that the combination improved recurrence-free survival in this patient population compared with placebo.
- In the final analysis of the COMBI-AD trial, researchers assessed overall survival and other survival metrics after more than 8 years of follow-up.
- This trial randomized 870 patients with resected stage III melanoma with BRAF V600 mutations to 150 mg twice daily dabrafenib plus 2 mg once daily trametinib or placebo for 12 months.
- The median duration of follow-up was 8.33 years for the combination therapy and 6.87 years for placebo.
TAKEAWAY:
- At 8 years, 71% of the patients who received dabrafenib plus trametinib survived vs 65% of those who received placebo, suggesting a 20% lower risk for death in the combination group (hazard ratio [HR], 0.80; 95% CI, 0.62-1.01). That overall survival benefit, however, did not reach statistical significance (P = .06).
- The greatest overall survival benefit was seen in patients with BRAF V600E–mutated melanoma (HR, 0.75; 95% CI, 0.58-0.96). This overall survival trend was reversed, however, in patients with BRAF V600K (HR, 1.95; 95% CI, 0.84-4.50).
- The combination also demonstrated better median melanoma-specific survival (HR, 0.78; 95% CI, 0.59-1.02) and continued to show a significant benefit in median recurrence-free survival (HR, 0.52; 95% CI, 0.43-0.63) and distant metastasis-free survival (HR, 0.56; 95% CI, 0.44-0.71).
- Overall, 97% of the patients in the combination group experienced an adverse event vs 88% in the placebo group. Serious adverse events occurred in 41% of the combination group vs 13% of the placebo group. The incidence of primary or secondary cancer was higher in the combination group as well — 3.98 events per 100 patient-years vs 2.61 in the placebo group.
IN PRACTICE:
One year of adjuvant therapy with dabrafenib plus trametinib was associated with a 20% lower risk for death than placebo — though the benefit was not significant — as well as a 25% lower risk for death among patients with a BRAF V600E mutation, the authors wrote.
SOURCE:
This study, with first author Georgina V. Long, MD, PhD, was published online last month in The New England Journal of Medicine.
LIMITATIONS:
The major limitations included inadequate power of the subgroup analyses to evaluate the effect of the treatment on overall survival and a poor comparator arm.
DISCLOSURES:
The study was funded by GlaxoSmithKline and Novartis. Four authors declared being employees or holding stocks of Novartis or GlaxoSmithKline. Several authors declared other industry ties.
